Carl R. Woese Institute for Genomic Biology

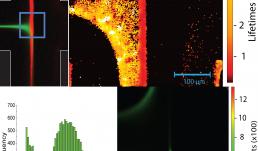
Flourescence lifetimes provide a valuable tool for distinguishing among fluorescing species where more tradition means such as intensity or spectral signature may be insufficient. In these images, a Zeiss LSM 710 microscope was used in conjunction with fluorescence lifetime imaging (FLIM) from ISS to measure lifetimes in-situ as an oligopeptides assembled in a microfluidic device. A pi-conjugated peryline-diimide (PDI) core is flanked by symmetric sequences of amino acids. These units self-assemble upon protonation at low pH. In the microfluidic device, coaxially focused flow of deionized water surrounding unassembled synthetic peptide is directed toward an opposed flow of 10 mM HCl, meeting in a cross-slot geometry. Planar extensional flow aligns oligopeptide structures as they assemble at the interface between these two flows. Assembled oligopeptide exhibits shorter lifetimes than unassembled. The lifetime image and corresponding histogram not only demonstrate the existence of distinct species, but also shows that the shorter lifetime material is indeed at the oligopeptide assembly front. The difference between the fluorescence lifetime map (top right) and intensity map (bottom right) demonstrating that fluorescence lifetime information can be used to inform the assembly process despite potentially uninformative fluorescence emission intensity data.


It has been shown that exposure to green (λ=550 nm, I=16 W/m2) fluorescent excitation light can induce force relaxation in living cells. Minimizing illumination time is critical to reducing light-induced force relaxation. We utilized a microscopic setup equipped with a mechanical shutter to evaluate the effect of short (<1s) exposure of living fibroblast cells to green light for one hour following the initial exposure. Cells were plated on polyacrylamide hydrogels embedded with dark red fluorescent nano particles which serve as fiducial markers. Motion of the nano particles was used to quantify change in cell force. Using a Zeiss Apotome Fluorescent Microscope with a Zeiss Axioobserver Z1 built-in reflected light shutter (<60 ms frequency), we exposed cells to green (λ=550 nm, I=16 W/m2) fluorescent excitation light for t=500 ms. Following this initial exposure, an image of the beads under each cell was captured every 5 minutes for one hour with dark red fluorescent excitation light (λ=650 nm, I=8.6 W/m2), which does not induce force changes. A widefield fluorescent metal halide lamp (X-Cite® Series 120Q) was used in conjunction with Semrock Brightline FP (green) and Cy5 (red) filters to illuminate the sample. The video shows the one-hour time lapse sped up 3000x (0.1s = 5 min in real time). The bead motion in the video illustrates that cells exposed to green light for only 500 ms do not exhibit widespread relaxation following exposure. The inward-outward-inward bead fluctuations show that localized cell forces continue to oscillate long after exposure to green fluorescent excitation light. Reducing exposure time to 500 ms may eradicate the widespread, light-induced force relaxation commonly observed in cells following initial illumination.
Watch the Video



This image, taken with the LSM 710 confocal microscope, shows two embryoid bodies (EBs), which are spherical aggregates of pluripotent cells that can be differentiated into the three germ layers. The EBs in this image are composed of mouse embryonic stem cells that have been differentiated into motor neurons (green) and glia (red); nuclei are stained in blue. Over time, the cells extend neurites across the underlying collagen substrate to form synapses with each other. This research is supported by an NSF-funded STC, Emergent Behaviors of Integrated Cellular Systems (www.ebics.net).



System: Imaging Cytometer (iCyte)
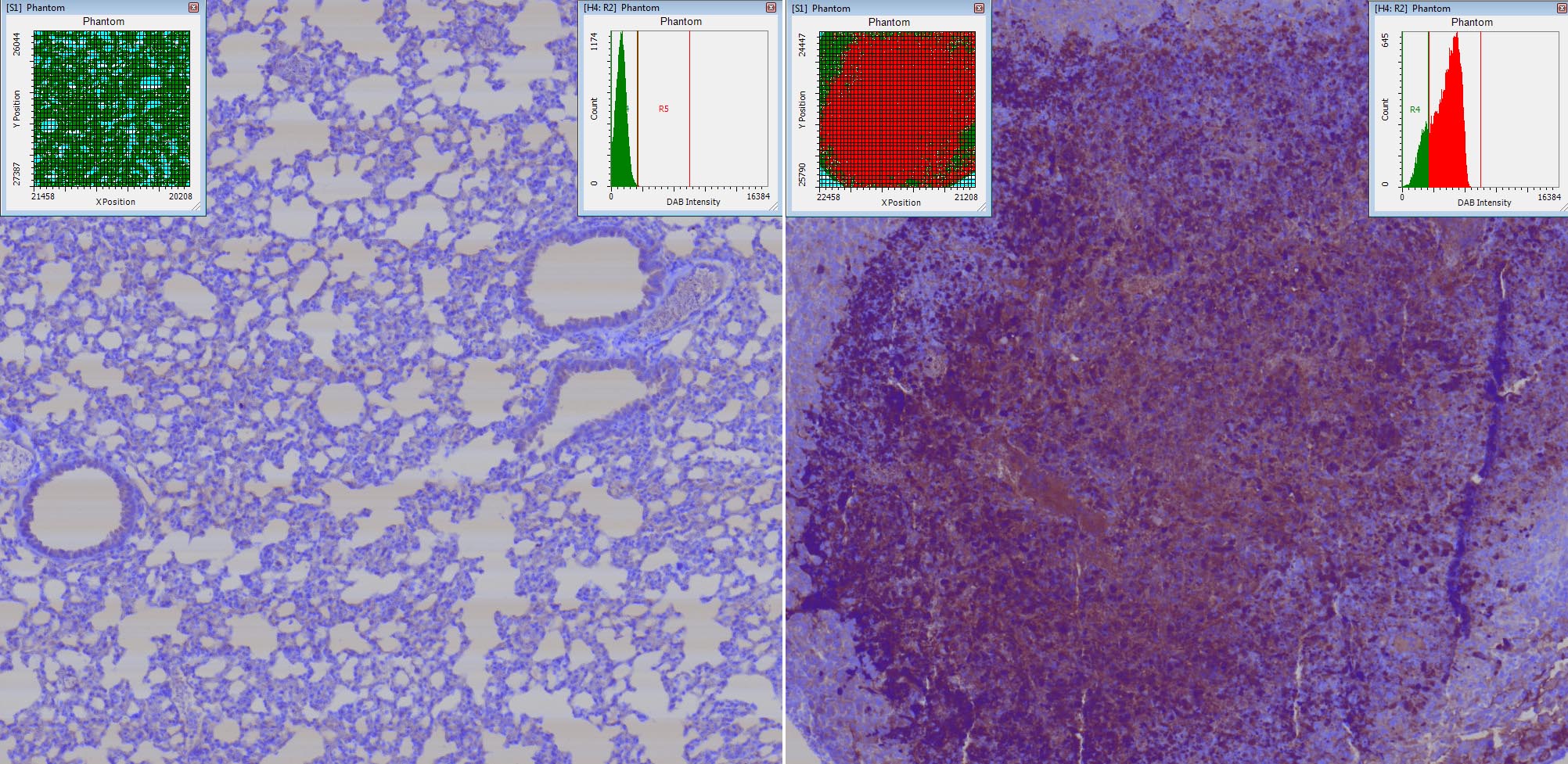
Caspase-3 DAB and Hematoxylin staining of tissue sections of mouse tumors. The iCyte allows you to quantify the signal intensity of Caspase-3 in tumor vs non tumor cells.
The iCyte is a laser scanning (automated and quantitative imaging) cytometer which combines digital microscopy with image processing and the real time population data analysis of analytical cytometry. It is a non-confocal system that utilizes laser-spot scanning illumination. The detection system, which combines photo PMTs for fluorescence detection and photodiodes (PD) for absorbance and scatter detection, offers excellent quantitative cytometric performance and imaging capabilities.
The instruments' unique design allows simultaneous use of fluorescent and chromatic dyes. There are numerous cellular applications of iCyte to areas of study including histochemistry, cell cycle/mitosis, DNA damage, apoptosis, cell-cell interactions, circulating tumor cells, siRNA, translocation/cell signaling etc.


System: Nanozoomer
Masson trichrome staining of chicken footpad skin. The footpad skin of chicken feet is covered with multiple reticular scales that have a papillary form. The structure of these papillae includes a thick outer layer called stratus corneum (brown) followed by epidermis (pink), dermis (blue), and subcutaneous tissue (blue and white). Chicken feet or paws have become the third most important economic part of the chicken, with chicken paws accounting for approximately $280 million a year. Footpad dermatitis (FPD) is a type of skin inflammation that causes necrotic lesions on the plantar surface of footpads in broilers and turkeys, which causes downgrades and condemnations of saleable chicken paws. Novus International is studying the structure integrity of footpad skin and pathology of FPD, in order to provide nutritional strategies to prevent and/or intervene development of footpad lesions and promote wound healing in commercial poultry.



U251 glioblastoma multiforme cells within a 3D gelatin hydrogel imaged using the LSM710 confocal microscope.



This image is tiled together from 16 individual photographs taken on the AxioZoom V16 microscope using cathodoluminescence stage. The specimen is a petrographic thin section of a fossiliferous limestone from Eastern New York. The various red, orange, and yellow colors represent different phases of calcite with different trace element compositions. Each phase represents a stage of formation or diagenesis in the limestone's history.



This is an image of a neuromuscular junction in a rat larynx, imaged with the LSM 710 confocal system and using two photon second harmonic generation imaging microscopy. The nerve terminal (red) attaches to the motor endplate (green) together with the muscle (grey SHG image) imaged with a GaAsP BiG detector without any label. This image shows how the neuromuscular junction interacts with the muscles of the vocal fold to cause contractions after receiving signals from the brain. By imaging the neuromuscular junctions with a variety of instruments including superresolution techniques, this project aimed to study the effects of age on neuromuscular junctions.


View Gallery
1
/
10
