Carl R. Woese Institute for Genomic Biology


This displays the 3D fused structure of two axial elements (AEs) during the diplotene stage of prophase I. The stained protein is SYCP3.
As the chromosomes progress through prophase I of meiosis I, the synaptonemal complex (SC) forms between homologous chromosomes to facilitate accurate chromosomal segregation. The complex is composed of two axial elements (AEs) ,SYCP2 and SYCP3, and a central element (CE). The CE is then linked to the AE via transverse filaments called SYCP1. As the chromosomes progress through diplotene, the SC begins to dissociate as the CE and SYCP1 disappear. In order to maintain the stability and integrity of the chromosomes, the AE migrate towards each other and undergo remodeling by fusing with each other.
The image was taken using 3D MINFLUX.
Contributors: Reza Rajabi-Toustani, Sasha Kakkassery, Huanyu Qiao


The image showcases regenerating tissue from Drosophila larval wing imaginal disc, captured in a top-down (XY) view alongside orthogonal views (YZ and XZ planes). The tissue is stained to highlight different structures: DAPI marks nuclei (blue), actin highlights the cytoskeleton (red), the plasma membrane is shown in magenta, and dying cells are labeled in green.
In the Smith-Bolton lab, we investigate tissue regeneration in Drosophila larval wing imaginal discs. This image focuses on the regenerating wing disc pouch, where cell debris (green) indicates areas of damage, and the regenerating tissue is prominently marked by actin (red), highlighting the structural changes during the repair process. Image taken using LSM900 with airyscan and joint deconvolution processing by Snigdha Mathure.


Atomic Force Microscopy reveals detailed nanoscale collagen fibril arrangement in pig tendon captured in a 4*4 μm scan. Fibril diameters range from 120 nm to 150 nm, with D-band periodicity approximately 67 nm. This precise measurement of fibril dimensions and their arrangement into fibers offers key insights into the structural pattern of collagenous tissues. Such understanding is crucial for pathological analysis, especially for looking at fibrotic conditions, where collagen deposition is a key factor. This work was done by Mahmuda Rakib Arshee from Amy Wagoner-Johnson lab. Image was taken with the Cypher AFM.
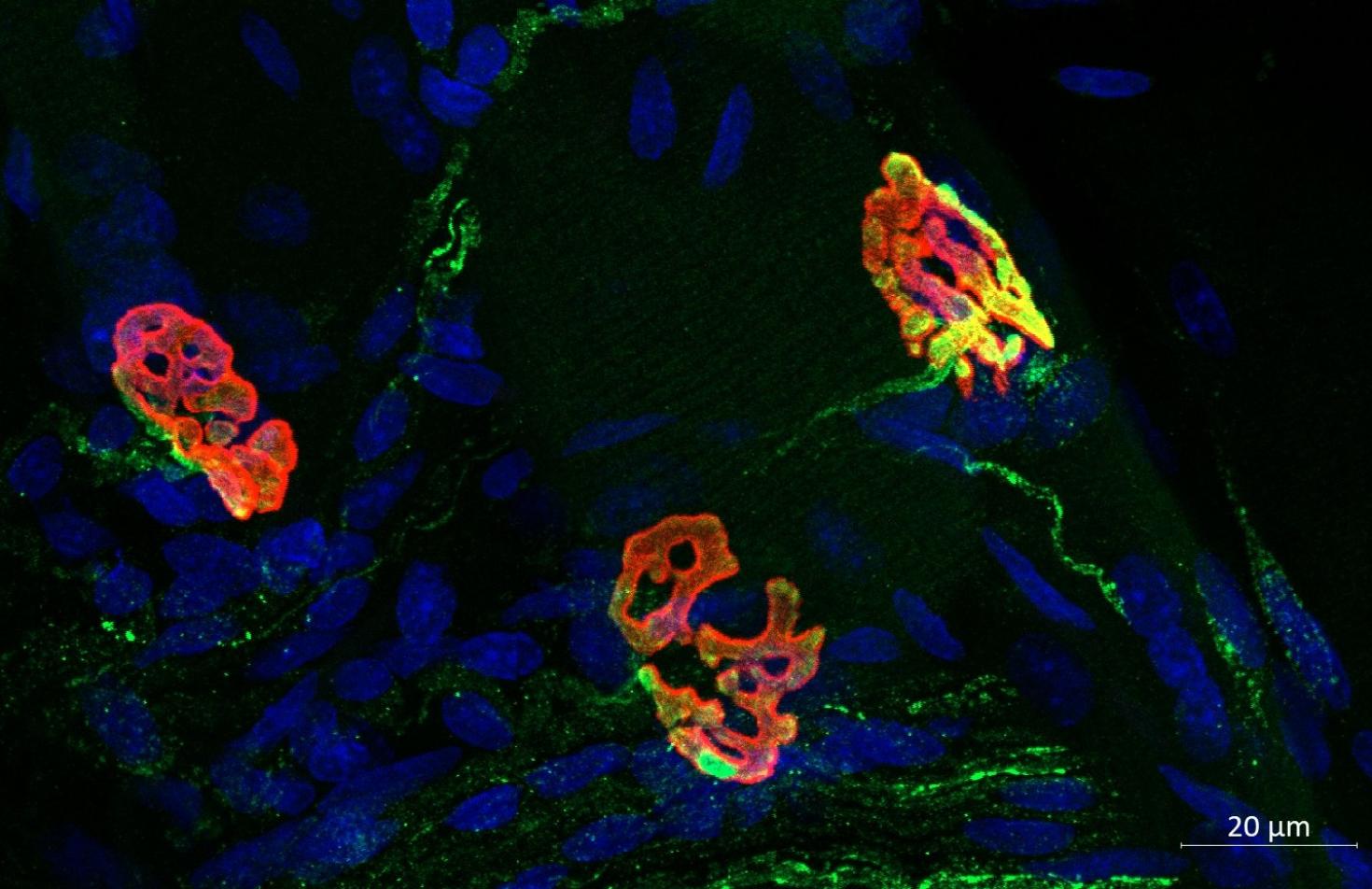
Three neuromuscular junctions (NMJs) from the diaphragm of a young male rat, illustrate the complex connections between motor neurons and muscle fibers that facilitate precise control of muscle contractions for breathing. This work was done by Sarah Asif from the Martha Gillette Lab and funded by the Chan Zuckerberg Biohub Chicago Acceleration Award. Image was taken with the LSM 900.
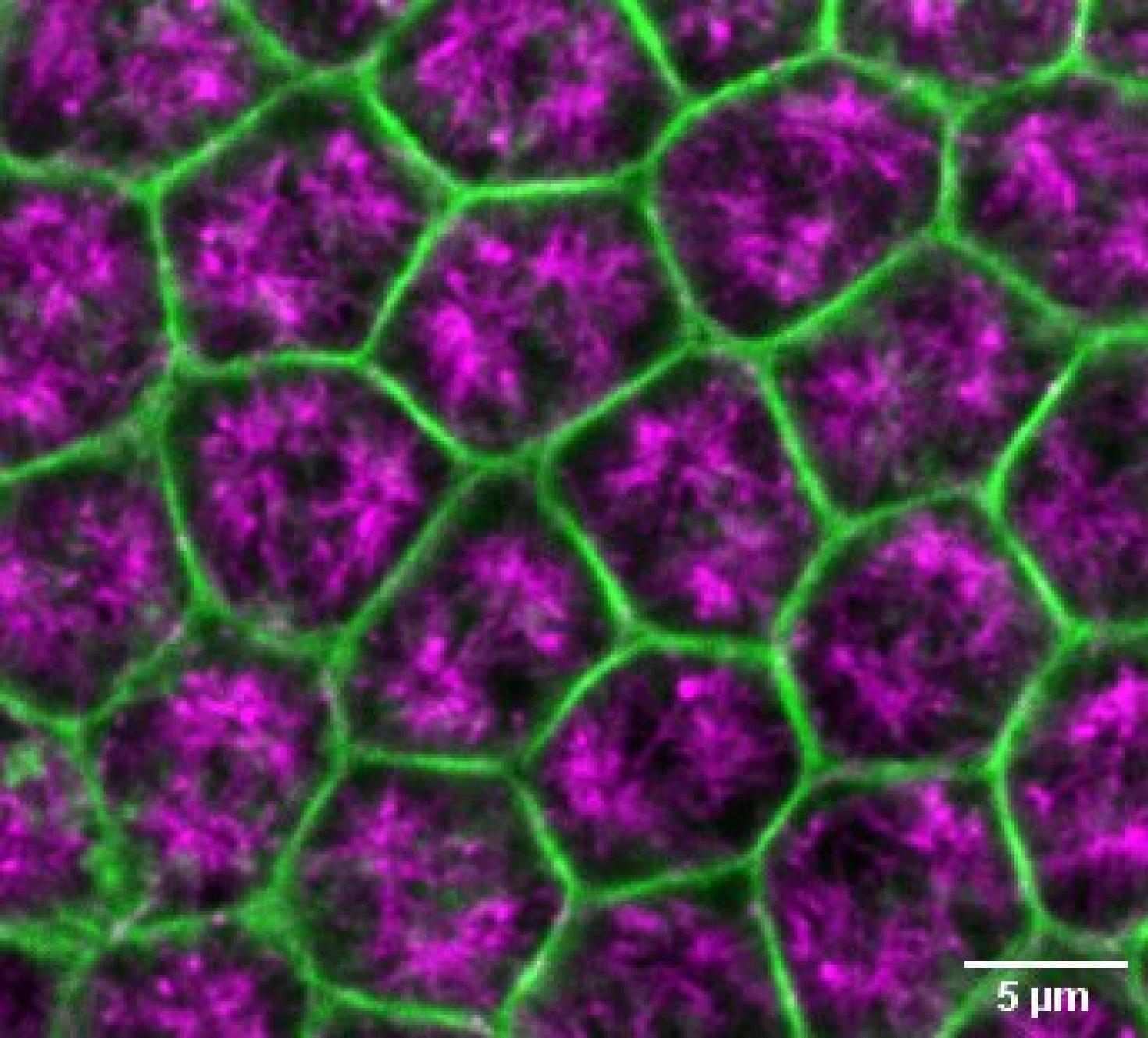
This image demonstrates a surface view of Drosophila embryo undergoing cellularization, depicting microtubule in magenta and Actin in green. The objective of this study is to investigate the changes in microtubule-actin interactions in the microvillar membrane reservoir during early embryonic morphogenesis in an intact tissue. The image was captured using STEDYCON microscope at the Core Facilities of the Carle R. Woese Institute for Genomic Biology.
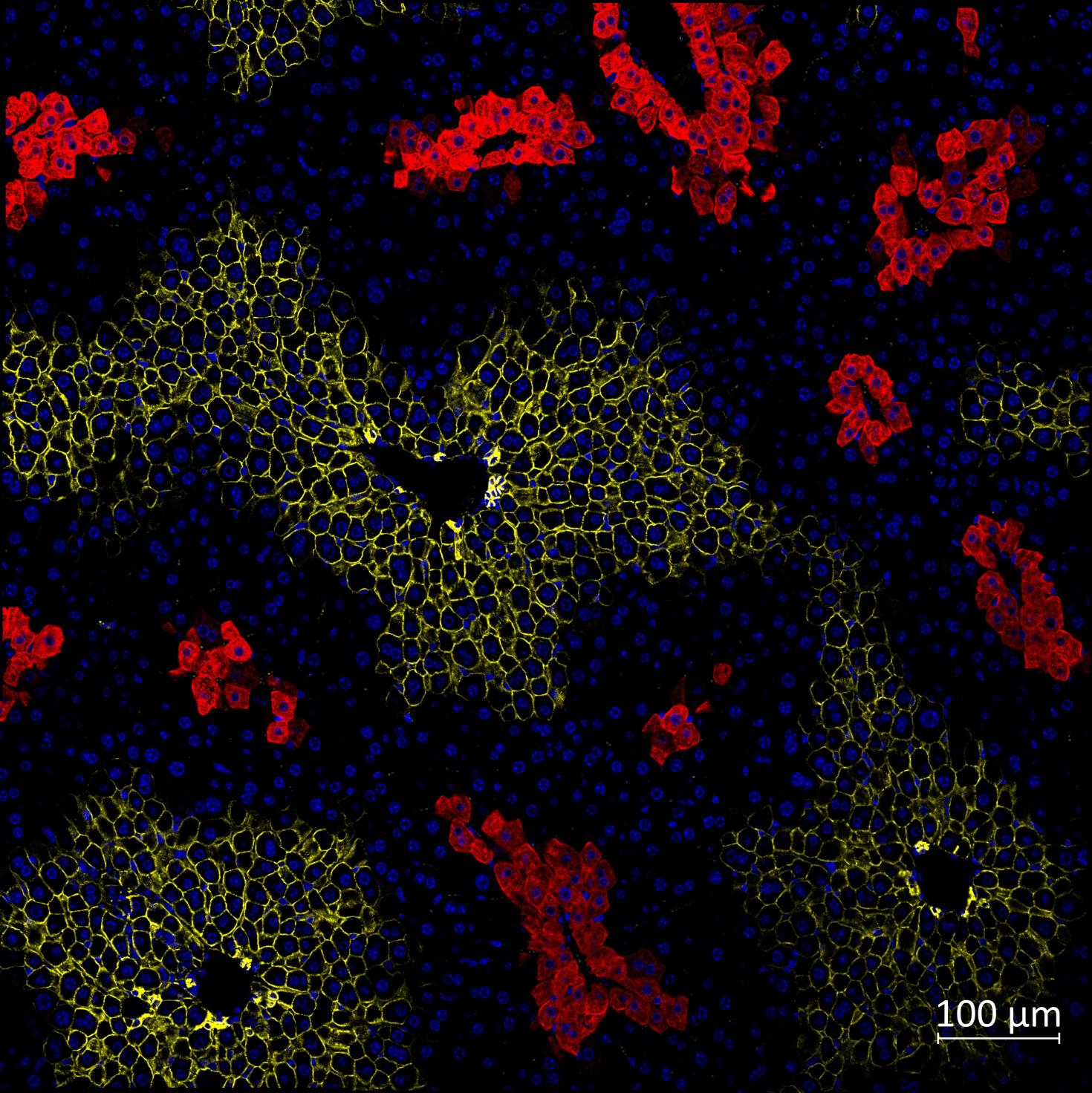
Immunofluorescence of mice liver sections with nuclei (blue) and zonal markers E-cadherin (periportal, yellow) and glutamine synthetase (pericentral, red). The liver helps maintain metabolic processes, hormone production, nutrient storage, and detoxification. This happens when the oxygen and nutrient-rich blood flow from the periportal vein to the pericentral vein in the liver lobule, the functional unit of liver. As a result, the blood flow creates a gradient of oxygen, nutrients and signaling molecules, creating three different zones in the liver lobule. These zones have different functions, and they express different proteins depending on their functions. Here, we have used Glutamine synthetase (red) to view the pericentral zone and E-cadherin (yellow) for the periportal zone to study the zonation.
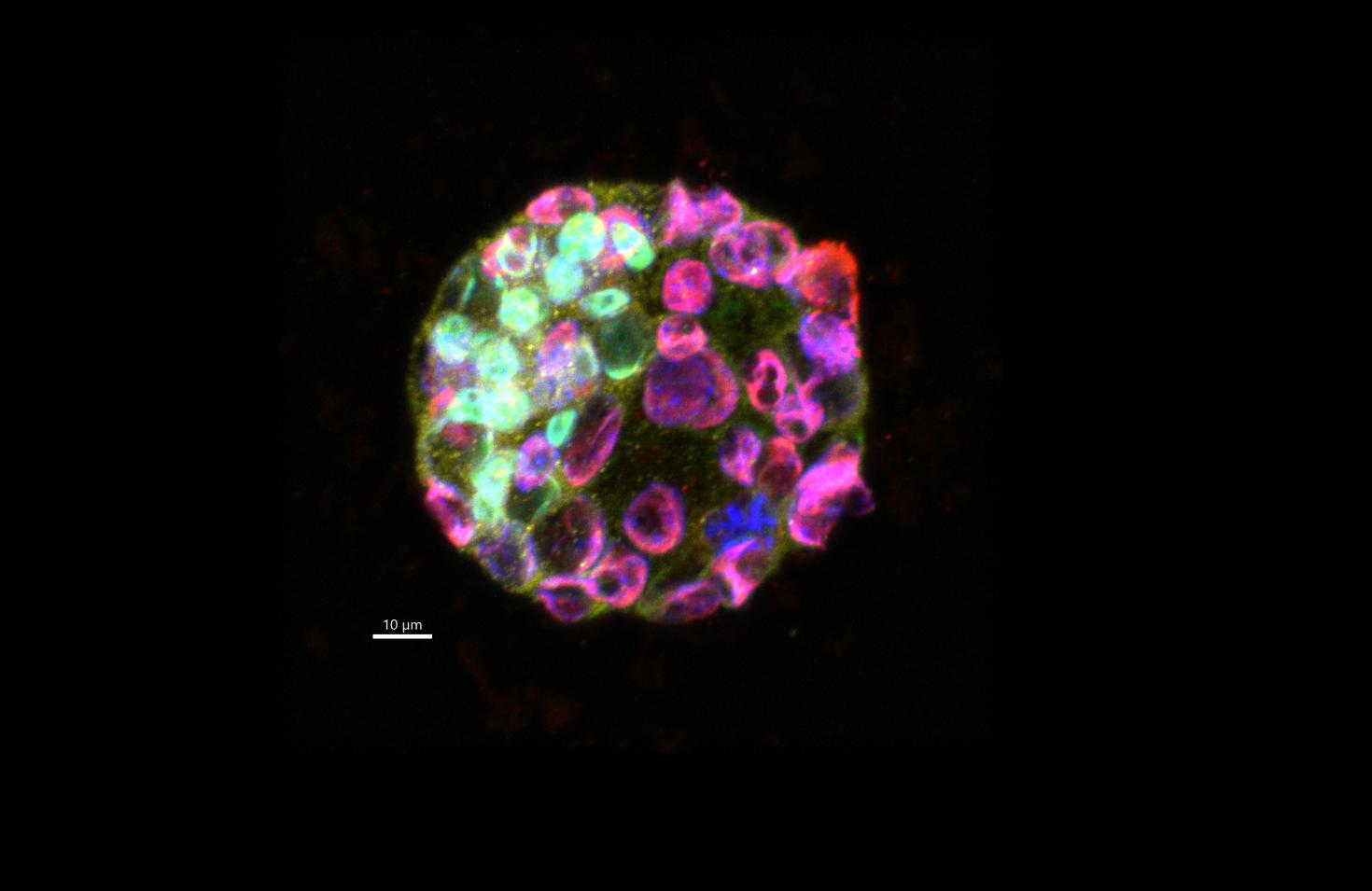
The proteins we evaluated in this embryo are GATA6 (green), CDX2 (red) and OCT4 (yellow). DAPI is shown in blue.
This embryo is part of a study to identify the effects of DEHP, which is a plasticizer whose chemical structure closely resembles hormones and is believed to be an endocrine disruptor. Previous studies have shown that it affects embryo development and implantation in the uterus. These proteins are important in cell differentiation to give rise to different organs in future stages. For this reason we want to evaluate its expression in the presence of DEHP and to understand the mechanism of its effect on embryo development and embryo implantation.
Image taken on LSM880.
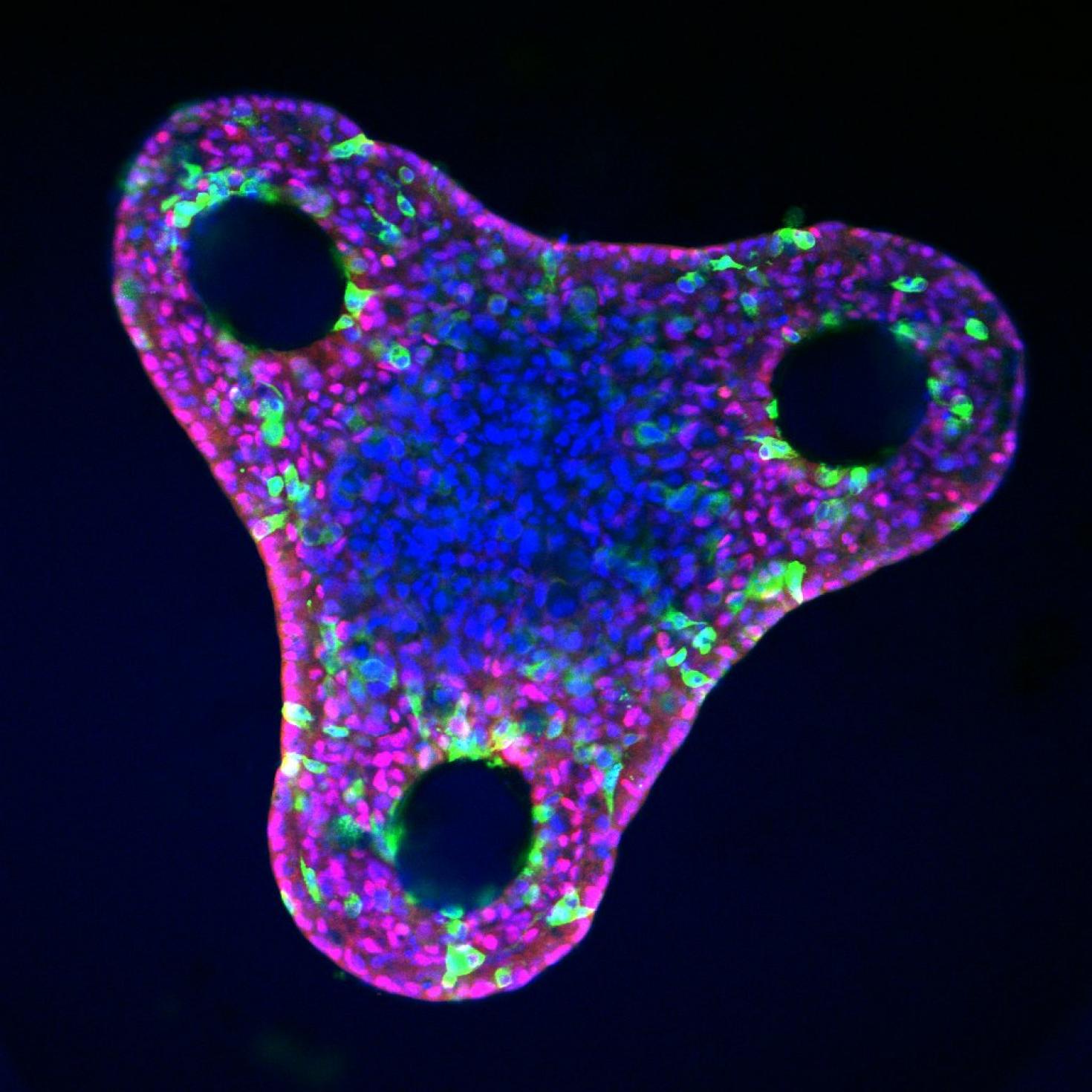

Liver organoid after differentiation in a triangular microwell. Varying organoid shape changes mechanical cues and can influence liver cell fate and patterning. The red stain is HNF4a, a hepatocyte marker, green is OPN, a biliary cell marker, and blue is DAPI, a nuclear DNA stain. Images were taken with the LSM 700.
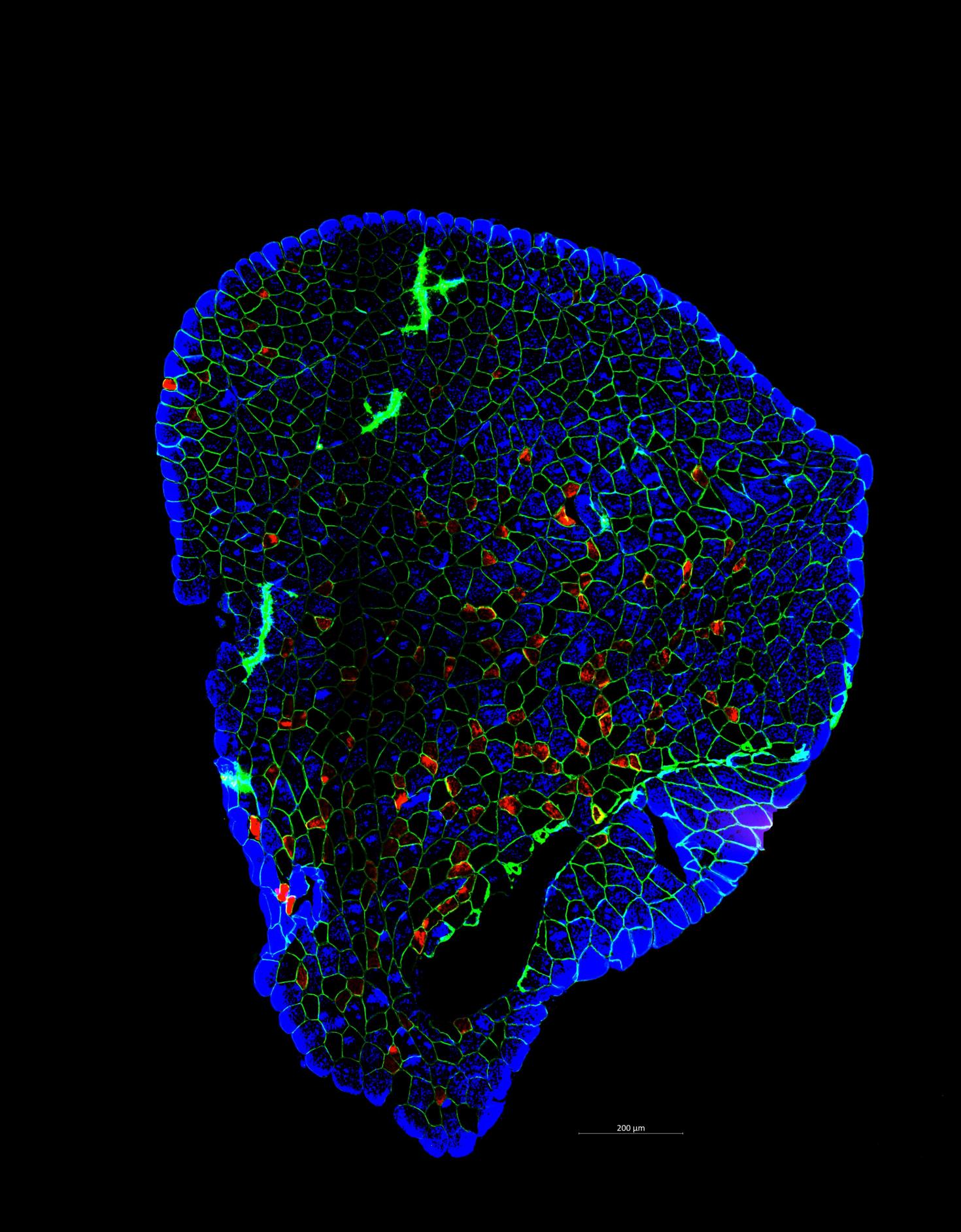
A plantaris muscle stained for individual fiber types. Fibers are "outlined" in green (stained for dystrophin). While the individual fibers are stained based on myosin heavy chain. Type IIa is red, IIx is black, and IIb is blue.
Instrument used: Axioscan
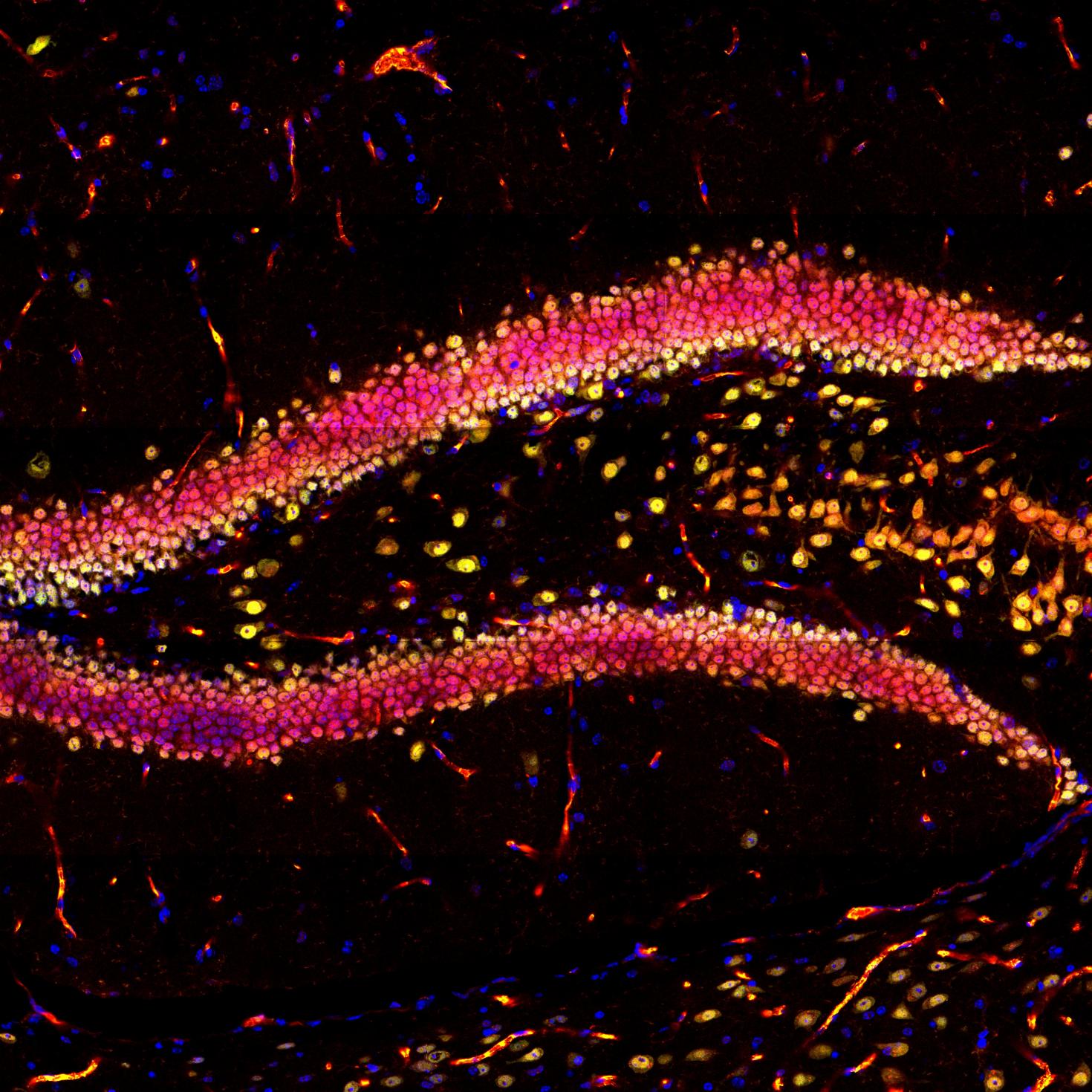

This image showcases the hippocampal region in a mouse brain slice, highlighting DAPI in blue, NeuN in green, and YAP in red using immunofluorescence. The objective of this research project is to investigate the impact of physical exercise on neurogenesis in the hippocampus. The images were captured using the Zeiss LSM 700 confocal microscope at the Core Facilities of the Carl R. Woese Institute for Genomic Biology.

View Gallery
1
/
10
