Moving from the visible to the infrared: developing high quality nanocrystals
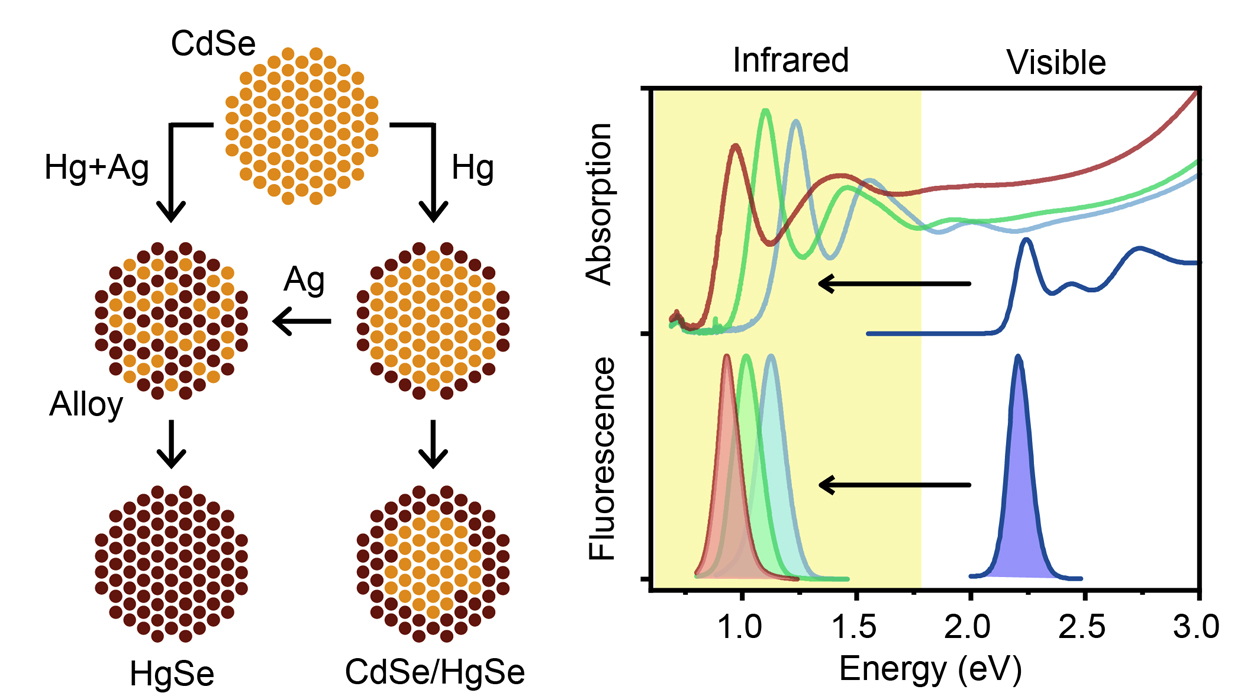
Schematic representation of CdSe nanocrystal exchange (left) and absorption spectra showing the shift of nanocrystals from the visible spectrum (CdSe) to the infrared (HgSe) (right). / Wonseok Lee
Awarded the 2023 Nobel Prize in Chemistry, quantum dots have a wide variety of applications ranging from displays and LED lights to chemical reaction catalysis and bioimaging. These semiconductor nanocrystals are so small—on the order of nanometers—that their properties, such as color, are size dependent, and they start to exhibit quantum properties. This technology has been really well developed, but only in the visible spectrum, leaving untapped opportunities for technologies in both the ultraviolet and infrared regions of the electromagnetic spectrum.
In new research published in Nature Synthesis, University of Illinois at Urbana-Champaign bioengineering professor Andrew Smith (CGD) and postdoctoral researcher Wonseok Lee have developed mercury selenide (HgSe) and mercury cadmium selenide (HgCdSe) nanocrystals that absorb and emit in the infrared, made from already well-developed, visible spectrum cadmium selenide (CdSe) precursors. The new nanocrystal products retained the desired properties of the parent CdSe nanocrystals, including size, shape and uniformity.
“This is the first example of infrared quantum dots that are at the same level of quality as the ones that are in the visible spectrum,” Smith says.
Although nanocrystal technology has existed for more than 50 years, only nanocrystals that operate in the visible portion of the spectrum have been significantly advanced. Smith explains, “They’re a big part of display devices. And a big part of any technology that is light absorbing or light emitting. There’s just been an intrinsic push to develop a technology that has the biggest market at the end of the day.”
Beyond just the market demand for visible spectrum nanocrystals, chemistry is harder for materials in the infrared, which is longer wavelength and lower energy than light in the visible spectrum. To achieve light absorption and emission in the infrared, heavier elements that are lower on the periodic table need to be used. Chemistry with those elements is more difficult, yielding more unwanted side reactions and less predictable reactions. They are also prone to degradation and are susceptible to ambient changes in the environment, like water.
Quantum dot nanocrystals can be made from elemental semiconductors, like silicon, or they can be binary or ternary. Mixing two elements can yield many different properties, mixing three elements together can yield exponentially more properties. “We have been focusing on this one type of material, a ternary alloy—mercury cadmium selenide—because we think it could be the ‘perfect’ material to make,” Smith says. “You could basically get any property you want by changing the ratio of cadmium and mercury atoms. It can span this huge range of the electromagnetic spectrum—across the entire infrared into the entire visible spectrum—and get so many properties.”
Smith had been trying to make this material since he was in graduate school with no luck, and even in the broader research community, there have been no reports of success, until now. “The way we did it was taking the already perfected, visible ones—cadmium selenide, which is considered to be the most developed quantum dot—and used it as a ‘sacrificial mold’,” he says.
Replacing the cadmium atoms with mercury atoms instantly shifts everything into the infrared spectrum, with all the desired quality retained: strong light absorption, strong light emission and homogeneity.
To do this, Smith and Lee had to ditch the traditional method of synthesis for nanocrystals, which is to mix the precursor elements together and under the right conditions, they decompose into the desired nanocrystal form. As it turns out, there are no conditions that anybody has found to work for mercury, cadmium and selenide.
“Lee developed a new process called interdiffusion enhanced cation exchange,” Smith says. “In this process, we add a fourth element, silver, which introduces defects in the material that causes everything to mix together homogeneously. And that solved the whole problem.”
While quantum dots have many applications, one application for infrared quantum dots with potential to have the most impact is for use as molecular probes for imaging, where they can be put into biological systems and detected in tissues. Since most quantum dots emit in the visible spectrum, only emission near the surface of the skin can be detected. Biology, however, is fairly transparent in the infrared and therefore deeper tissues can be probed.
Mice are the standard models for most diseases and Smith explains that with quantum dots that emit in the infrared, researchers could see almost entirely through a living rodent to see its physiology and the locations of specific molecules throughout the body. This will allow for better understanding of biological processes and for developing therapeutics without having to sacrifice the mice, potentially changing preclinical drug development.
---
Andrew Smith is also an affiliate of the Holonyak Micro & Nano Technology laboratory, the department of materials science & engineering, the Cancer Center at Illinois and the Carle Illinois College of Medicine at Illinois.
Wonseok Lee is also an affiliate of the Holonyak Micro & Nano Technology laboratory at Illinois.
This research was funded by the National Institutes of Health and the National Science Foundation.
