Carl R. Woese Institute for Genomic Biology

This is a newly hatched first instar honey bee larva. The goal of this project is to develop a system to rear honey bees in the laboratory using advanced robotics. Once established, the system will be used to study how parasites, pathogens, pesticides and nutrition interact to affect bee health. This research is supported by a grant from DARPA and is conducted by Julia Fine, William Streyer, Ran Chao and Nathan Beach in the laboratory of Gene Robinson. The image was captured on a Zeiss AxioZoom V16.


Autofluorescence image of a human kidney stone collected from a patient using percutaneous nephrolithotomy (PCNL) under sterile operating room conditions at the Mayo Clinic in Rochester, Minnesota. The sample is comprised of a complex crystalline growth sequence of hydroxyapatite, calcium oxalate monohydrate, calcium oxalate dihydrate and uric acid. A 30 micron-thick polished slice of the kidney stone was imaged on a Zeiss LSM 880 Airyscan microscope. This tiled super-resolution image was obtained using three fluorescence channels, with excitation wavelengths of 488, 561 and 633 nm. Bright red fluorescence indicates areas where epoxy fills void spaces, while all other emissions are caused by emissions from organic material entombed within the kidney stone crystals. This image indicates that several unexpected events took place during the formation of the kidney stone, which include: (1) multiple events of wholesale mineral dissolution and recrystallization; (2) mobilization and re-assembly of multiple stone fragments; (3) ultra nanometer-scale layering; and (4) entombment and preservation of microbial biofilms.
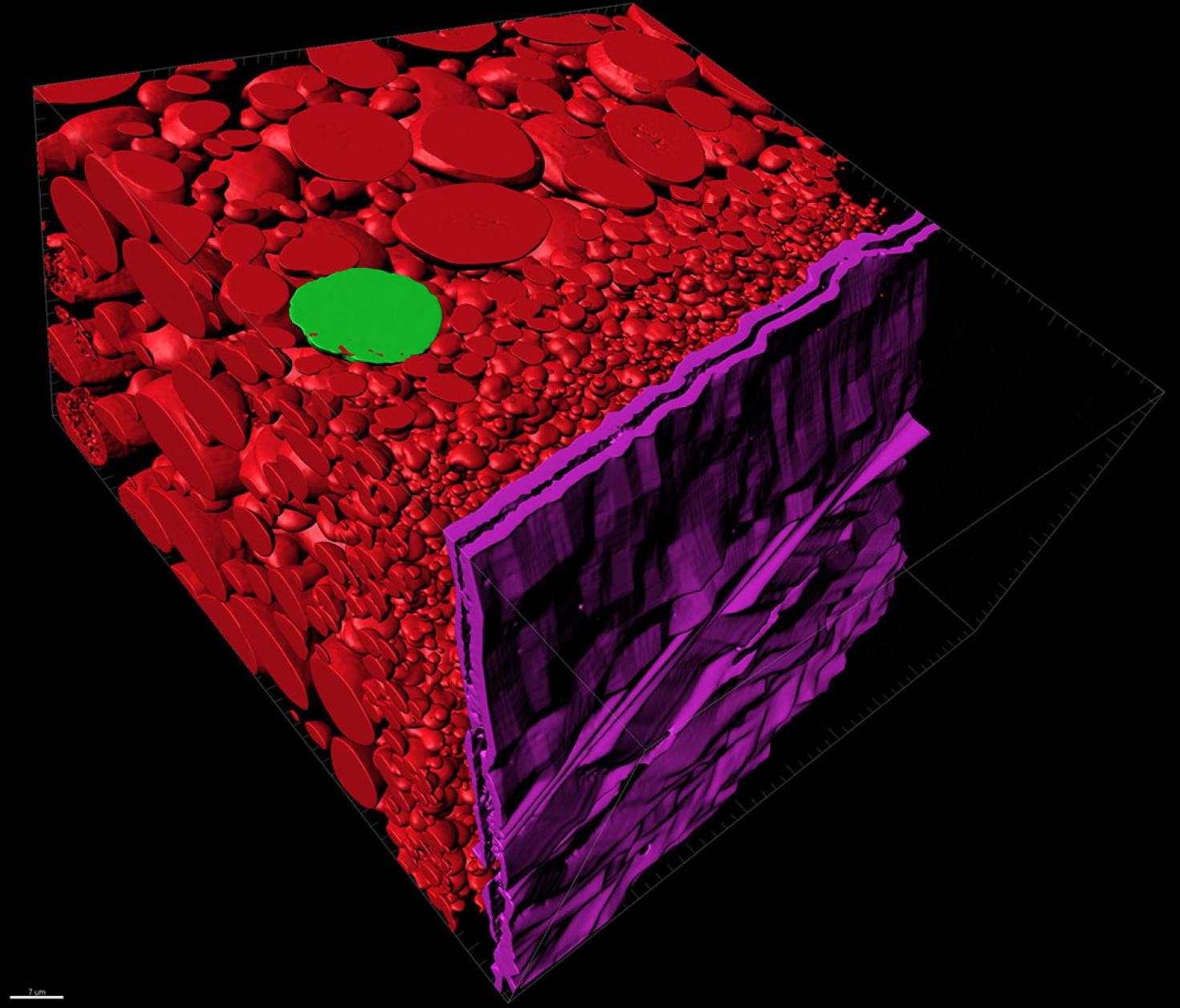

Scattered across this image are segmented cross sections of a clawed frog egg. The image was taken with a Zeiss Sigma VP 3View Serial Block-Face SEM (SBF-SEM), segmented in Microscopy Image Browser and imported into Imaris to create a surface. The goal of the segmentation was to measure the individual volumes of the different sized yolk. The large red circles mark the yolk substance that feeds the developing embryo. The violet segmented structure represents the two-layered walls of the yolk. Clawed frogs are often used as an experimental model for studies like this one that help us understand how organisms develop.
The Jing Yang Lab is interested in germ plasm in Xenopus oocytes which are organized into small "islands" and dispersed to the vegetal cortex of the oocyte. After fertilization, these small islands come together and form very large aggregates. As the embryo divide, a few cells inherit these large germ plasm aggregates and become primordial germ cells. The Lab is interested in the mechanisms controlling these processes. In oocytes and early embryos, these germ plasms are located vegetally, very close to the cortex of the oocyte or embryo. It contains large amounts of mitochondrial, ER, and insoluble large protein complexes. It can be easily identified by TEM.
The aim of using SBF-SEM was to study how the germ plasm dynamics are regulated at the molecular level. The Lab is currently studying some factors which are likely important for germ plasm dynamics and plans to overexpress or knockdown some genes of interest and study how the manipulation would affect either the localization/organization of germ plasm.


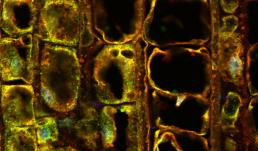
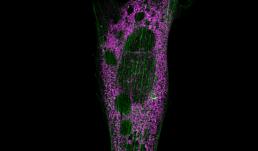
Eastern Filbert Blight is a fungal disease that attacks European hazelnut trees. The disease is challenging to manage in part because it takes months for symptoms to appear after infection. The red spine-like structures in this image are the growing filaments of the fungus invading a plant stem, shown in green with a tangle of red fibers from the plant's vascular tissue. Documenting the fungal growth pattern will help plant biologists diagnose and control the disease.


Coral skeletons are made up of the mineral Aragonite, which typically has needle and fan shaped crystals. The nanozoomer microscope typically has light paths for brightfield and fluorescence modalities. In this image, we have created a new modality in this system by utilizing a set of a polarizer and analyzer in the brightfield path at an orientation which is not typically used in polarized microscopy (crossed nicols). An addition of another 90 degree rotation provides both brightfield as well as polarization like image which provide an isotropic visualization of all crystals partially illuminated by brightfield light and the rest showing polarization extinctions illustrated by a range of colors.

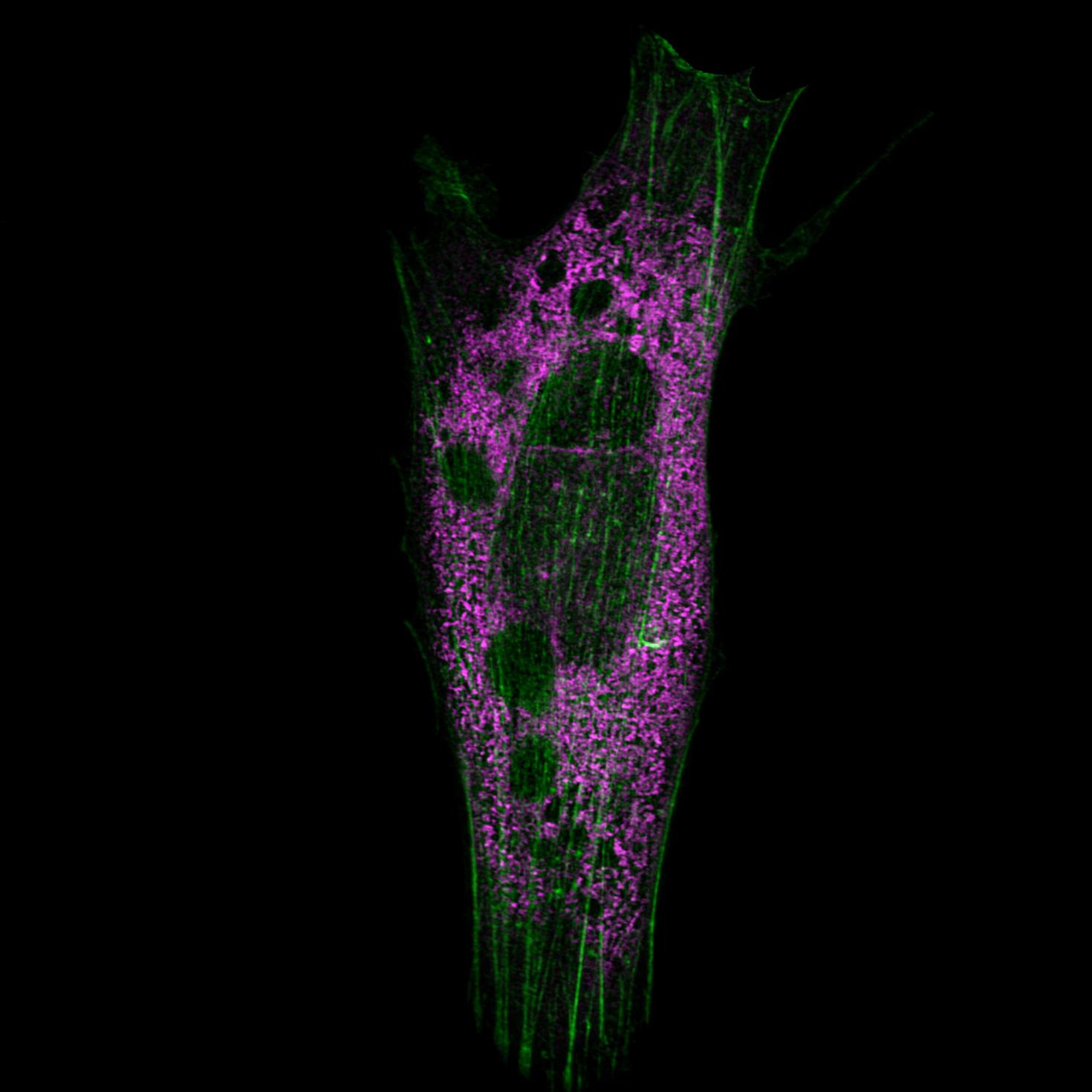
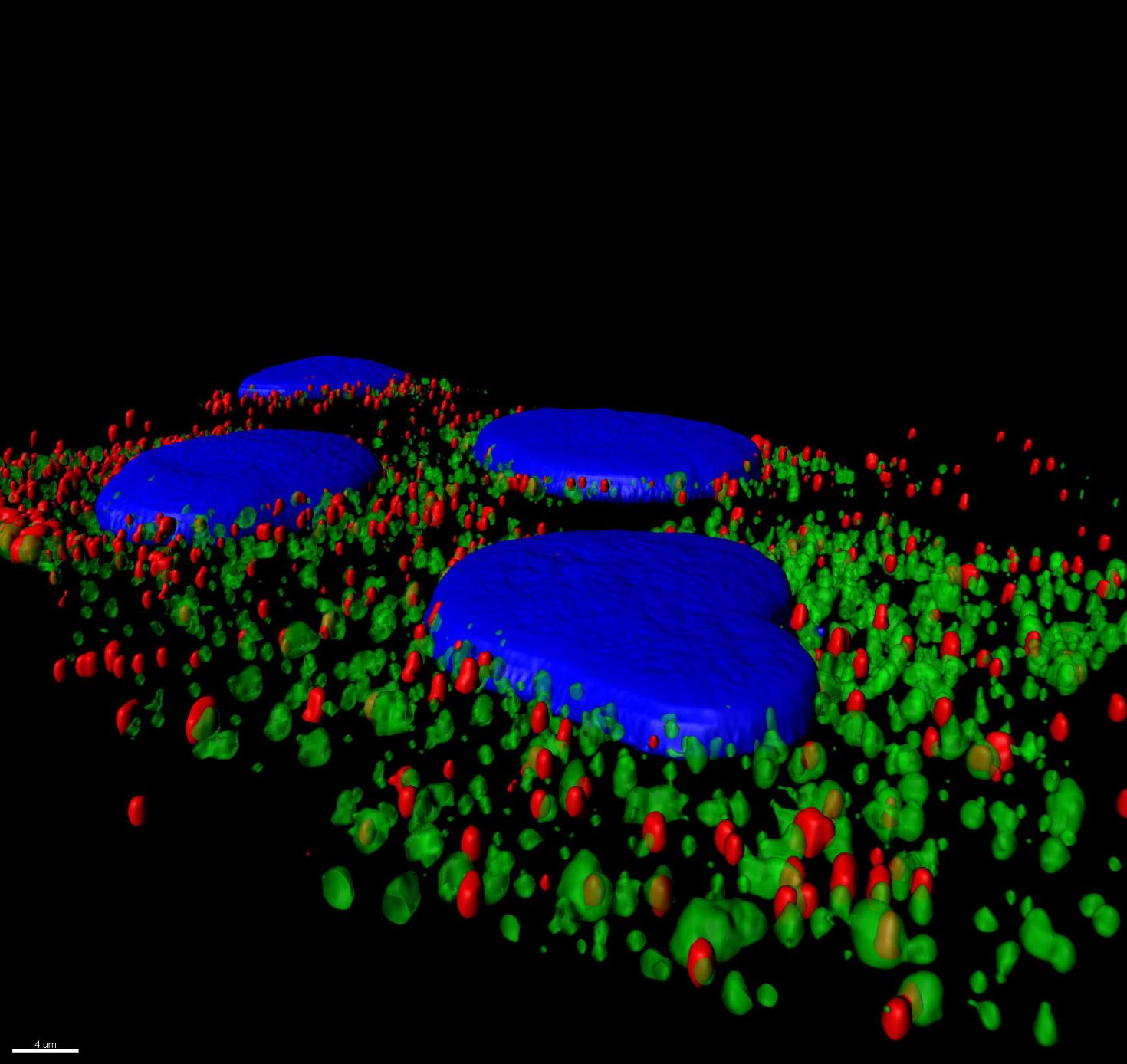
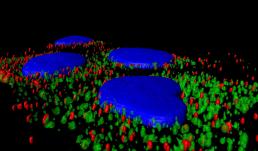
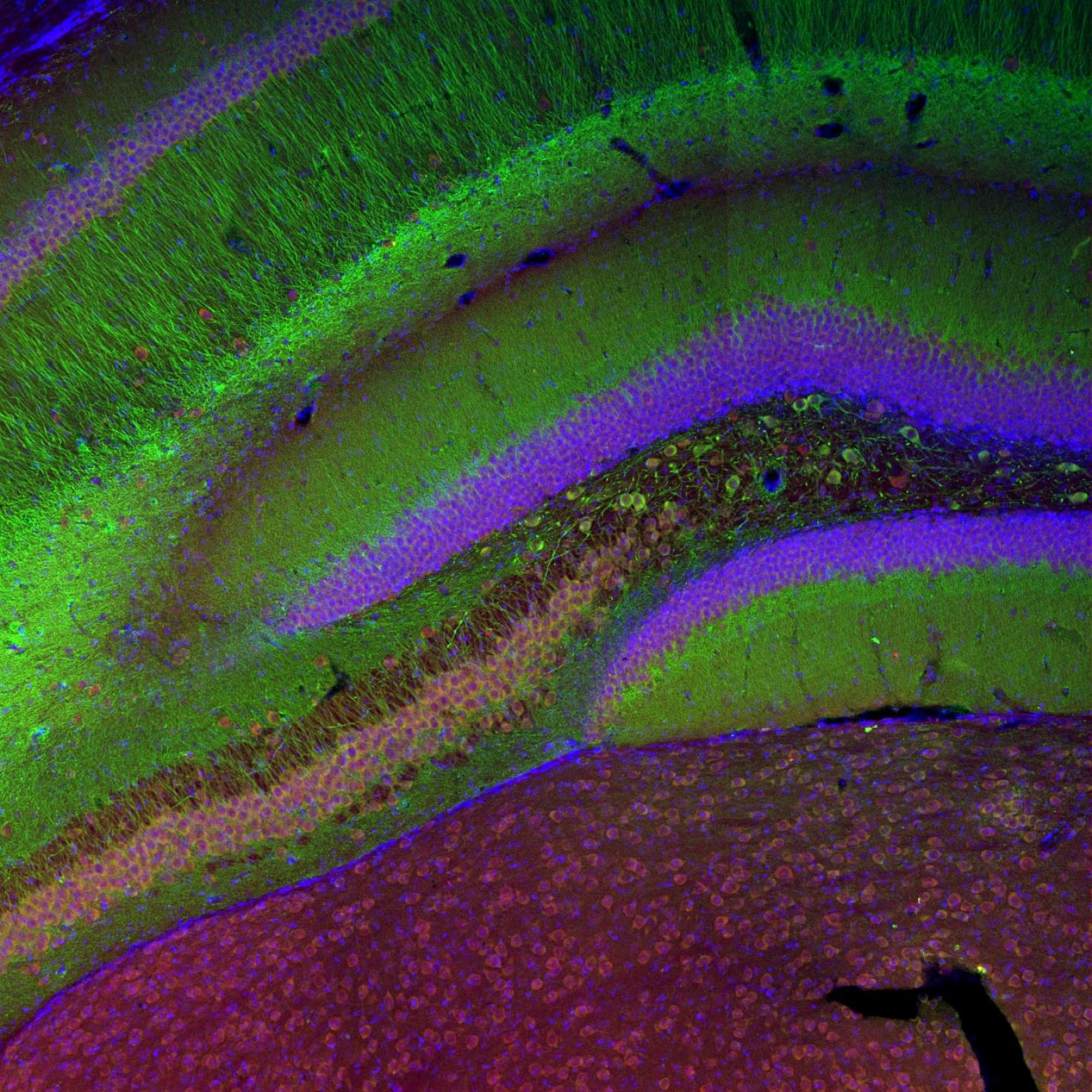
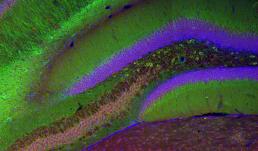
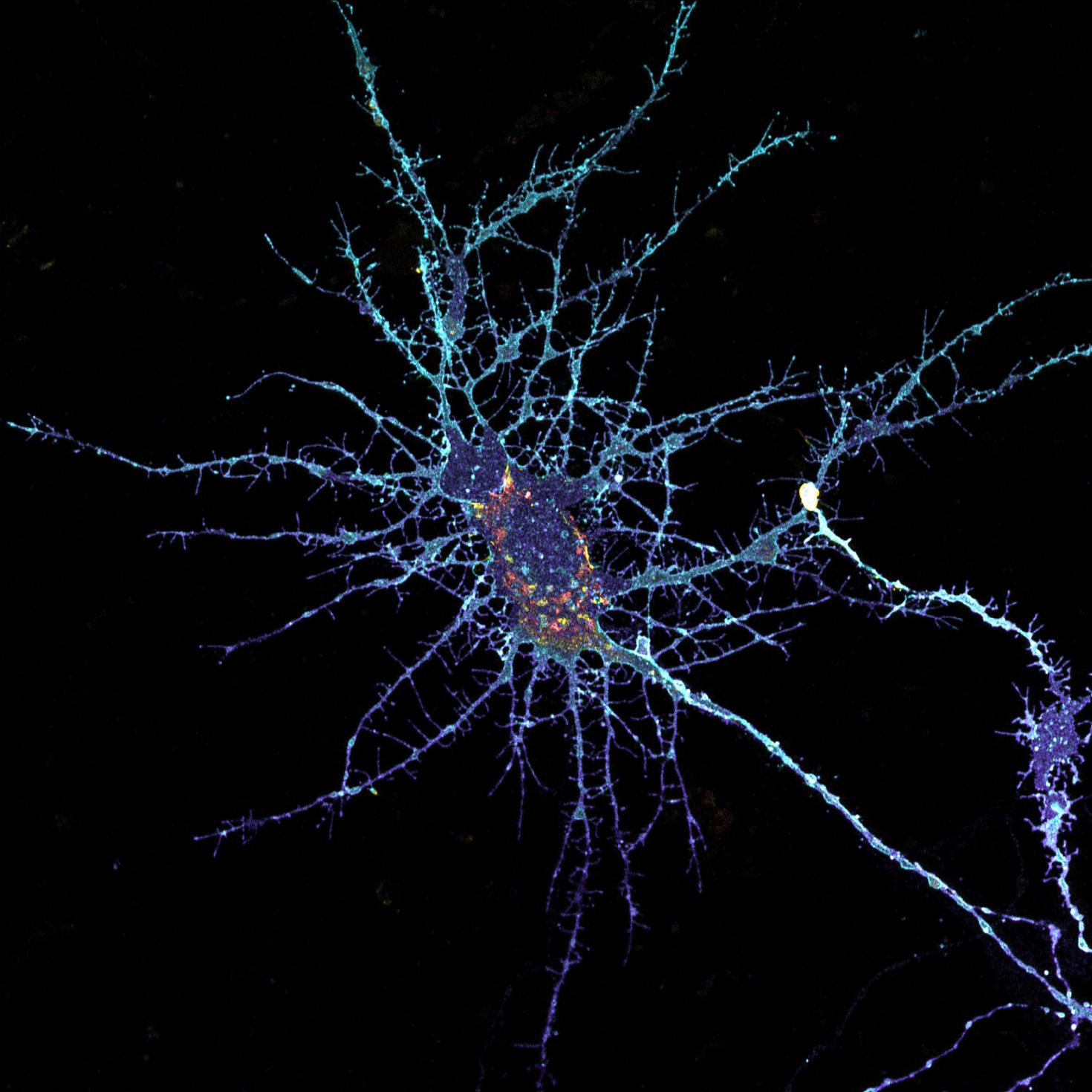
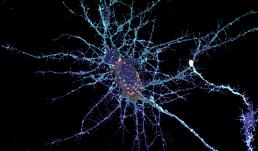
Neurons form complex networks. Of particular interest are dendrites, which receive signals from other neurons. They form extensive branched structures that are self-organized. This is achieved with the help of filopodia, which are finger-like projections from dendrites that sense the microenvironment and guide decisions on branch formation and synapse formation. We used the LSM Fast Mode to study how inhibiting miR-125b affects the structure of dendritic filopodia and dendrites themselves. Here, cultured hippocampal neurons are treated with a peptide nucleic acid (PNA) inhibitor of miR-125b, and transfected with a BacMam 2.0 cell membrane-GFP virus. This allows us to visualize the morphology of these neurons, and specifically study features of dendrites and filopodia at a high resolution.

View Gallery
1
/
10
